[vc_row][vc_column][vc_column_text css=””]Vitamin B12, also known as cobalamin, is an essential nutrient vital for a wide range of bodily functions. It plays a crucial role in DNA synthesis, nerve function, and the production of red blood cells. When it comes to B12 supplementation, two common forms stand out: cyanocobalamin and methylcobalamin. Understanding the differences between these two forms is crucial for making informed choices about your health. In this comprehensive article, we’ll delve into the chemical structure, bioavailability, potential benefits, and safety aspects of cyanocobalamin and methylcobalamin.
Chemical Structure: Cyanocobalamin vs. Methylcobalamin
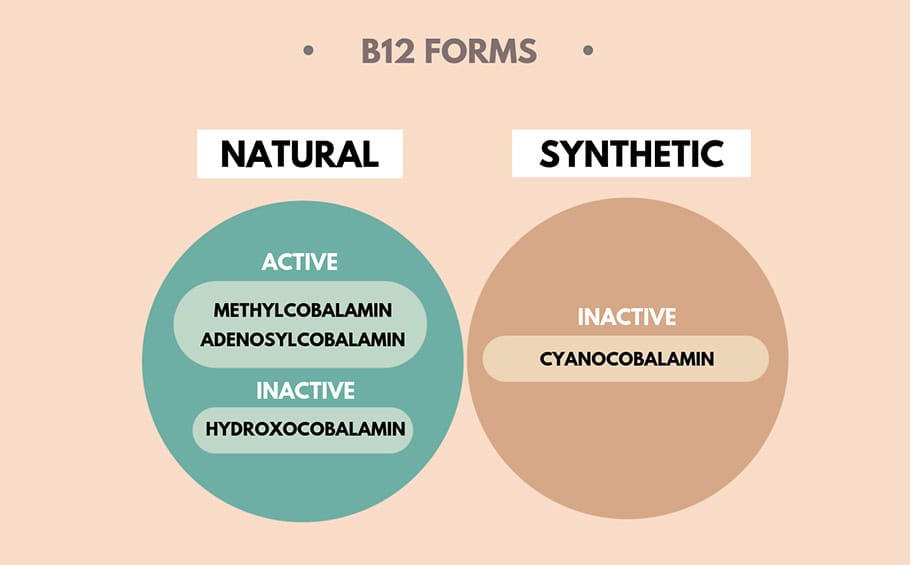
Cyanocobalamin: Cyanocobalamin is a synthetic form of vitamin B12. It is composed of a cobalamin molecule with a cyanide group (CN) attached. While the inclusion of cyanide might raise concerns, it’s essential to understand that the amount is minuscule and considered safe for most people. This form of vitamin B12 is highly stable and less expensive to produce, making it the most common form used in supplements and fortified foods.
Methylcobalamin: Methylcobalamin is the naturally occurring form of vitamin B12. It contains a methyl group (CH3) linked to the cobalamin molecule. This methyl group is crucial for various biochemical reactions in the body, making methylcobalamin an essential coenzyme for these processes. Due to its natural form, it is considered more bioavailable and suitable for specific health concerns.
Bioavailability: A Key Distinction
Bioavailability refers to the extent and rate at which a substance can be absorbed and used by the body. It’s a critical factor when comparing cyanocobalamin and methylcobalamin.
Cyanocobalamin: Cyanocobalamin is not directly usable by the body. It needs to undergo a conversion process to become metabolically active. Enzymes within the body facilitate this conversion. Although this process is generally efficient for most individuals, it does mean that cyanocobalamin is considered slightly less bioavailable compared to the active forms of B12, such as methylcobalamin.
Methylcobalamin: Methylcobalamin, in contrast, is already in the biologically active form. This means that when you take methylcobalamin, your body can immediately utilize it for various essential functions. The absence of conversion makes it the most bioavailable form of vitamin B12, providing a distinct advantage for those with specific B12 needs.
Potential Benefits of Cyanocobalamin and Methylcobalamin
Both cyanocobalamin and methylcobalamin offer a range of potential benefits, although they may be better suited for different purposes.
Cyanocobalamin:
- Treatment of B12 Deficiency: Cyanocobalamin is effective in addressing B12 deficiencies and preventing conditions like pernicious anemia. It is commonly used in clinical settings for injections and is found in many over-the-counter B12 supplements.
- Cost-Effectiveness: Cyanocobalamin is often less expensive to produce, making it a more economical option for B12 supplementation.
Methylcobalamin:
- Neurological Support: Methylcobalamin is believed to be more effective in supporting nerve health. It has been studied for its potential benefits in conditions like diabetic neuropathy. More research is needed, but the early findings are promising.
- Direct Bioavailability: Its immediate usability by the body makes methylcobalamin a preferred choice for those with specific health concerns or who desire rapid B12 absorption.
Safety Considerations
Both cyanocobalamin and methylcobalamin are generally safe when used as directed. However, there have been concerns about cyanocobalamin due to its cyanide content. It’s important to emphasize that the cyanide content is extremely low and not typically a concern in standard doses. If you have specific health conditions or concerns, it’s always advisable to consult with a healthcare professional before starting any supplement regimen.
How is Cyanocobalamin made?
Cyanocobalamin, the industrially produced form of vitamin B12, is primarily manufactured through a microbial fermentation process. Here are the key steps involved in the production of cyanocobalamin:
Microorganism Selection
– The most commonly used microorganisms for industrial cyanocobalamin production are strains of Propionibacterium freudenreichii and Pseudomonas denitrificans[1][3].
– Other strains like Pseudomonas nitroreducens and Ensifer (Sinorhizobium) fredii have also been employed[3].
Fermentation Process
– The fermentation process typically takes 3-7 days and is carried out in large bioreactors (>100,000 L)[1][4].
– The medium used contains a carbon source (usually glucose), nitrogen source, cobalt, and other essential nutrients[1][3].
– Optimizing parameters like temperature, pH, aeration, and agitation is crucial for maximizing cyanocobalamin yield[1][3].
Downstream Processing
1. **Extraction**: The broth is heated (80-120°C) at pH 6.5-8.5 to extract all corrinoids (forms of vitamin B12)[3].
2. **Cyanidation**: Potassium cyanide or thiocyanate is added to convert the extracted corrinoids into cyanocobalamin[3].
3. **Filtration and Adsorption**: The solution undergoes clarification by microfiltration and/or nanofiltration, followed by adsorption on resins like XAD to purify the cyanocobalamin[3].
4. **Further Purification**: For pharmaceutical-grade cyanocobalamin, additional adsorption steps using resins like IRA and alumina may be performed[3].
5. **Crystallization**: The purified cyanocobalamin is finally crystallized to obtain the final product[1][3].
Environmental Considerations
– Excess cobalt in the fermentation medium can lead to harmful waste products, so optimizing cobalt supply is important for sustainability[4].
– Researchers have developed a ‘metalation calculator’ to determine the optimal cobalt requirements for large-scale cyanocobalamin production[4].
In summary, industrial cyanocobalamin production relies on microbial fermentation, followed by a series of extraction, purification, and crystallization steps to obtain the final product. Ongoing research aims to make the process more efficient and environmentally friendly.
Citations:
[1] https://www.youtube.com/watch?v=9u6tS2-7HFo
[2] https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/cyanocobalamin
[3] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9405231/
[4] https://quadram.ac.uk/case_studies/an-environmentally-friendly-vitamin-b12-production-method-that-makes-manufacture-more-affordable/
[5] https://microbialcellfactories.biomedcentral.com/articles/10.1186/s12934-014-0102-7
How is Methylcobalamin made?
Based on the search results, methylcobalamin can be produced by the methylation of cyanocobalamin or hydroxocobalamin using dimethyl carbonate in the presence of a reducing agent:
1. **Cyanocobalamin (II) or hydroxocobalamin (III)** is methylated with dimethyl carbonate (IV) in the presence of a reducing agent (V) like sodium borohydride.
2. The reaction occurs in an aqueous or hydroorganic medium at mild conditions (5-60°C, slightly basic pH).
3. A sequestering agent for the cyanide ion, such as ferrous sulfate or cobalt chloride, is often used to improve the yield and selectivity.
4. The amount of dimethyl carbonate used ranges from 1 to 25 equivalents, while the reducing agent is used in 4 to 25 equivalents.
5. Under these conditions, methylcobalamin (I) is obtained with good yield and purity, without forming undesirable byproducts.
So in summary, the key steps are:
1. **Reduction** of cyanocobalamin/hydroxocobalamin
2. **Methylation** with dimethyl carbonate
3. **Purification** of the methylcobalamin product
The process allows the selective production of methylcobalamin under mild, aqueous conditions using readily available reagents like sodium borohydride and dimethyl carbonate. The presence of a cyanide scavenger helps optimize the yield.
Citations:
[1] https://www.sumobrain.com/patents/wipo/Process-production-methylcobalamin/WO2006100059A1.html
[2] https://patents.google.com/patent/EP1394174A1/en
[3] https://data.epo.org/publication-server/rest/v1.0/publication-dates/20060301/patents/EP1236737NWB1/document.pdf
[4] https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/methylcobalamin
[5] https://www.youtube.com/watch?v=9u6tS2-7HFo
The Bottom Line
In the debate of cyanocobalamin vs. methylcobalamin, the choice depends on your individual needs and preferences. If you have a vitamin B12 deficiency or seek a cost-effective way to maintain B12 levels, cyanocobalamin is a suitable option. On the other hand, if you’re targeting neurological health or desire the direct bioavailability of B12, methylcobalamin may be more fitting. While both forms of vitamin B12 are effective in treating deficiencies, methylcobalamin is often favored for its immediate bioavailability, neurological support, and longer retention in the body, making it a preferred choice for those with specific health needs. However, individual requirements and health conditions should always be considered when selecting a vitamin B12 supplement.
Ultimately, the importance of vitamin B12 in maintaining your health cannot be understated. Whether you opt for cyanocobalamin or methylcobalamin, both forms offer the promise of improved well-being, provided you make an informed choice based on your unique requirements. Always consult with a healthcare professional for personalized advice on your B12 supplementation journey.[/vc_column_text][/vc_column][/vc_row][vc_row][vc_column][vc_btn title=”Book Your B12 Injection Online Now!” color=”primary” size=”lg” align=”center” i_icon_fontawesome=”far fa-calendar” css=”” button_block=”true” add_icon=”true” link=”url:https%3A%2F%2Fcindywvet.setmore.com%2Fbeta%2Fservices%2F2c859ae4-7e14-473c-a123-187cd34b37a0%3Fsource%3Deasyshare|title:b12%20shot”][vc_empty_space][/vc_column][/vc_row]









